E. Lerner, E. Ploetz, J. Hohlbein, T. Cordes, S. Weiss, The Journal of Physical Chemistry B, 120, 6401–6410, 2016, [link]
Single molecule protein induced fluorescence enhancement (PIFE) serves as a molecular ruler at molecular distances inaccessible to other spectroscopic rulers such as Förster-type resonance energy transfer (FRET) or photo-induced electron transfer. In order to provide two simultaneous measurements of two distances on different molecular length scales for the analysis of macromolecular complexes, we and others recently combined measurements of PIFE and FRET (PIFE-FRET) on the single molecule level. PIFE relies on steric hindrance of the fluorophore Cy3, which is covalently attached to a biomolecule of interest, to rotate out of an excited-state trans isomer to the cis isomer through a 90 deg intermediate. In this work, we provide a theoretical framework that accounts for relevant photophysical and kinetic parameters of PIFE-FRET, show how this framework allows the extraction of the fold-decrease in isomerization mobility from experimental data and how these results provide information on changes in the accessible volume of Cy3. The utility of this model is then demonstrated for experimental results on PIFE-FRET measurement of different protein-DNA interactions. The proposed model and extracted parameters could serve as a benchmark to allow quantitative comparison of PIFE effects in different biological systems.

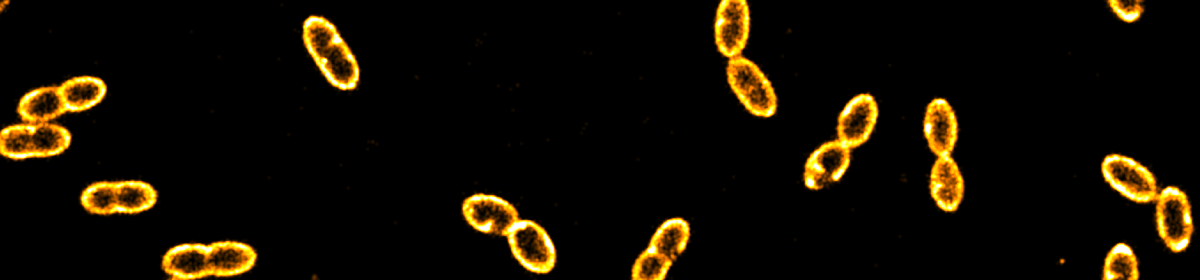
 he method is based on the detection of single photons, it additionally allows for performing fluorescence correlation spectroscopy (FCS) as well as dynamical anisotropy measurements thereby providing access to fast orientational dynamics down to the nanosecond time scale. The 3D orientation is particularly interesting in non-isotropic environments such as lipid membranes, which are of great importance in biology. We used giant unilamellar vesicles (GUVs) labeled with fluorescent dyes down to a single molecule concentration as a model system for both, assessing the robustness of the orientation determination at different timescales and quantifying the associated errors. The vesicles provide a well-defined spherical surface, thus, the in cooperation of lipid dyes (DiO) represents a a wide range of dipole orientations. To complement our experimental data, we performed Monte Carlo simulations of the rotational dynamics of dipoles incorporated into lipid membranes. Our study offers a comprehensive view on the dye orientation behavior in a lipid membrane with high spatiotemporal resolution representing a six-dimensional fluorescence detection approach.
he method is based on the detection of single photons, it additionally allows for performing fluorescence correlation spectroscopy (FCS) as well as dynamical anisotropy measurements thereby providing access to fast orientational dynamics down to the nanosecond time scale. The 3D orientation is particularly interesting in non-isotropic environments such as lipid membranes, which are of great importance in biology. We used giant unilamellar vesicles (GUVs) labeled with fluorescent dyes down to a single molecule concentration as a model system for both, assessing the robustness of the orientation determination at different timescales and quantifying the associated errors. The vesicles provide a well-defined spherical surface, thus, the in cooperation of lipid dyes (DiO) represents a a wide range of dipole orientations. To complement our experimental data, we performed Monte Carlo simulations of the rotational dynamics of dipoles incorporated into lipid membranes. Our study offers a comprehensive view on the dye orientation behavior in a lipid membrane with high spatiotemporal resolution representing a six-dimensional fluorescence detection approach.  preparation and characterization of two types of complex coacervate core micelles (C3Ms) with cross-linked cores and spectroscopic labels, and demonstrate their use as diffusional probes to investigate the microstructure of percolating biopolymer networks. The first type consists of poly(allylamine hydrochloride) (PAH) and poly(ethylene oxide)-poly(methacrylic acid) (PEO-b-PMAA), labeled with ATTO 488 fluorescent dyes. We show that the size of these probes can be tuned by choosing the length of the PEO-PMAA chains. ATTO 488-labeled PEO113-PMAA15 micelles are very bright with 18 dye molecules incorporated into their cores. The second type is a 19F-labeled micelle, for which we used PAH and a 19F-labeled diblock copolymer tailor-made from poly(ethylene oxide) poly(acrylic acid) (mPEO79-b-PAA14). These micelles contain approximately 4 wt% of 19F and can be detected by 19F NMR. The 19F labels are placed at the end of a small spacer to allow for the necessary rotational mobility. We used these ATTO- and 19F-labeled micelles to probe the microstructures of a transient gel (xanthan gum) and a cross-linked, heterogeneous gel (kappa-carrageenan). For the transient gel, sensitive optical diffusometry methods, including fluorescence correlation spectroscopy, fluorescence recovery after photobleaching and super-resolution single nanoparticle tracking allowed us to measure the diffusion coefficient in networks with increasing density. From these measurements, we determined the diameters of the constituent xanthan fibers. In the heterogeneous kappa-carrageenan gels, bi-modal nanoparticle diffusion was observed, which is a signpost of microstructural heterogeneity of the network.
preparation and characterization of two types of complex coacervate core micelles (C3Ms) with cross-linked cores and spectroscopic labels, and demonstrate their use as diffusional probes to investigate the microstructure of percolating biopolymer networks. The first type consists of poly(allylamine hydrochloride) (PAH) and poly(ethylene oxide)-poly(methacrylic acid) (PEO-b-PMAA), labeled with ATTO 488 fluorescent dyes. We show that the size of these probes can be tuned by choosing the length of the PEO-PMAA chains. ATTO 488-labeled PEO113-PMAA15 micelles are very bright with 18 dye molecules incorporated into their cores. The second type is a 19F-labeled micelle, for which we used PAH and a 19F-labeled diblock copolymer tailor-made from poly(ethylene oxide) poly(acrylic acid) (mPEO79-b-PAA14). These micelles contain approximately 4 wt% of 19F and can be detected by 19F NMR. The 19F labels are placed at the end of a small spacer to allow for the necessary rotational mobility. We used these ATTO- and 19F-labeled micelles to probe the microstructures of a transient gel (xanthan gum) and a cross-linked, heterogeneous gel (kappa-carrageenan). For the transient gel, sensitive optical diffusometry methods, including fluorescence correlation spectroscopy, fluorescence recovery after photobleaching and super-resolution single nanoparticle tracking allowed us to measure the diffusion coefficient in networks with increasing density. From these measurements, we determined the diameters of the constituent xanthan fibers. In the heterogeneous kappa-carrageenan gels, bi-modal nanoparticle diffusion was observed, which is a signpost of microstructural heterogeneity of the network.