N. Bourouina, D. de Kort, F. Hoeben, H. Janssen, H. Van As, J. Hohlbein, J. van Duynhoven, J.M. Kleijn, Langmuir, 31, 12635-12643, 2015, [link]
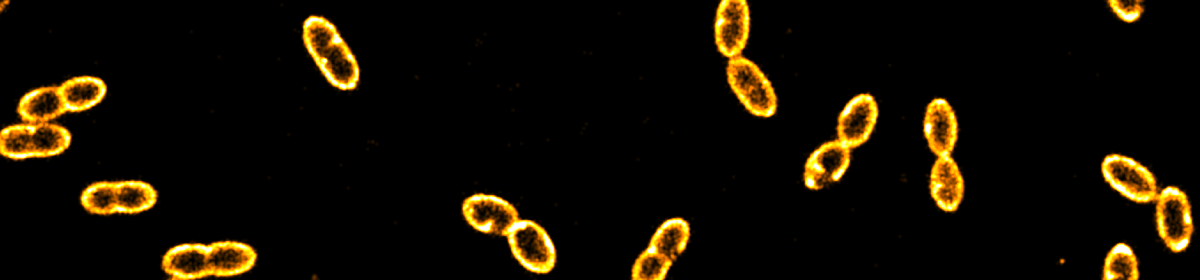
We present the design,  preparation and characterization of two types of complex coacervate core micelles (C3Ms) with cross-linked cores and spectroscopic labels, and demonstrate their use as diffusional probes to investigate the microstructure of percolating biopolymer networks. The first type consists of poly(allylamine hydrochloride) (PAH) and poly(ethylene oxide)-poly(methacrylic acid) (PEO-b-PMAA), labeled with ATTO 488 fluorescent dyes. We show that the size of these probes can be tuned by choosing the length of the PEO-PMAA chains. ATTO 488-labeled PEO113-PMAA15 micelles are very bright with 18 dye molecules incorporated into their cores. The second type is a 19F-labeled micelle, for which we used PAH and a 19F-labeled diblock copolymer tailor-made from poly(ethylene oxide) poly(acrylic acid) (mPEO79-b-PAA14). These micelles contain approximately 4 wt% of 19F and can be detected by 19F NMR. The 19F labels are placed at the end of a small spacer to allow for the necessary rotational mobility. We used these ATTO- and 19F-labeled micelles to probe the microstructures of a transient gel (xanthan gum) and a cross-linked, heterogeneous gel (kappa-carrageenan). For the transient gel, sensitive optical diffusometry methods, including fluorescence correlation spectroscopy, fluorescence recovery after photobleaching and super-resolution single nanoparticle tracking allowed us to measure the diffusion coefficient in networks with increasing density. From these measurements, we determined the diameters of the constituent xanthan fibers. In the heterogeneous kappa-carrageenan gels, bi-modal nanoparticle diffusion was observed, which is a signpost of microstructural heterogeneity of the network.
preparation and characterization of two types of complex coacervate core micelles (C3Ms) with cross-linked cores and spectroscopic labels, and demonstrate their use as diffusional probes to investigate the microstructure of percolating biopolymer networks. The first type consists of poly(allylamine hydrochloride) (PAH) and poly(ethylene oxide)-poly(methacrylic acid) (PEO-b-PMAA), labeled with ATTO 488 fluorescent dyes. We show that the size of these probes can be tuned by choosing the length of the PEO-PMAA chains. ATTO 488-labeled PEO113-PMAA15 micelles are very bright with 18 dye molecules incorporated into their cores. The second type is a 19F-labeled micelle, for which we used PAH and a 19F-labeled diblock copolymer tailor-made from poly(ethylene oxide) poly(acrylic acid) (mPEO79-b-PAA14). These micelles contain approximately 4 wt% of 19F and can be detected by 19F NMR. The 19F labels are placed at the end of a small spacer to allow for the necessary rotational mobility. We used these ATTO- and 19F-labeled micelles to probe the microstructures of a transient gel (xanthan gum) and a cross-linked, heterogeneous gel (kappa-carrageenan). For the transient gel, sensitive optical diffusometry methods, including fluorescence correlation spectroscopy, fluorescence recovery after photobleaching and super-resolution single nanoparticle tracking allowed us to measure the diffusion coefficient in networks with increasing density. From these measurements, we determined the diameters of the constituent xanthan fibers. In the heterogeneous kappa-carrageenan gels, bi-modal nanoparticle diffusion was observed, which is a signpost of microstructural heterogeneity of the network.

 mic integrity by copying DNA with high fidelity. A conformational change important for fidelity is the motion of the polymerase fingers subdomain from an open to a closed conformation upon binding of a complementary
mic integrity by copying DNA with high fidelity. A conformational change important for fidelity is the motion of the polymerase fingers subdomain from an open to a closed conformation upon binding of a complementary resonance
resonance  fluidic device based on syringe-driven flow of fluorescent species through a parallel array of nanochannels, in which the geometrical confinement enables long observation times of non-immobilized molecules. Extremely low flow rates are achieved by operating the array of nanochannels in parallel with a larger microchannel. The addition of a second microfluidic inlet allows for mixing different species in a well-defined volume, enabling the study of irreversible reactions such as DNA synthesis in real-time using single-molecule fluorescence resonance energy transfer. Devices are fabricated in glass with the purpose of high-throughput single-molecule fluorescence detection.
fluidic device based on syringe-driven flow of fluorescent species through a parallel array of nanochannels, in which the geometrical confinement enables long observation times of non-immobilized molecules. Extremely low flow rates are achieved by operating the array of nanochannels in parallel with a larger microchannel. The addition of a second microfluidic inlet allows for mixing different species in a well-defined volume, enabling the study of irreversible reactions such as DNA synthesis in real-time using single-molecule fluorescence resonance energy transfer. Devices are fabricated in glass with the purpose of high-throughput single-molecule fluorescence detection. scheme continues to expand the possibilities of fluorescence-based assays to study biological entities and interactions. Especially the combination of ALEX and single-molecule Förster Resonance Energy Transfer (smFRET) has been very successful as ALEX enables the sorting of fluorescently labelled species based on the number and type of fluorophores present. ALEX also provides a convenient way of accessing the correction factors necessary for determining accurate molecular distances. Here, we provide a comprehensive overview of the concept and current applications of ALEX and we explicitly discuss how to obtain fully corrected distance information across the entire FRET range. We also present new ideas for applications of ALEX which will push the limits of smFRET-based experiments in terms of temporal and spatial resolution for the study of complex biological systems.
scheme continues to expand the possibilities of fluorescence-based assays to study biological entities and interactions. Especially the combination of ALEX and single-molecule Förster Resonance Energy Transfer (smFRET) has been very successful as ALEX enables the sorting of fluorescently labelled species based on the number and type of fluorophores present. ALEX also provides a convenient way of accessing the correction factors necessary for determining accurate molecular distances. Here, we provide a comprehensive overview of the concept and current applications of ALEX and we explicitly discuss how to obtain fully corrected distance information across the entire FRET range. We also present new ideas for applications of ALEX which will push the limits of smFRET-based experiments in terms of temporal and spatial resolution for the study of complex biological systems. ymerases depends on conformational changes that promote the rejection of incorrect nucleotides before phosphoryl transfer. Here, we combine single-molecule FRET with the use of DNA polymerase I and various fidelity mutants to highlight mechanisms by which active-site side chains influence the conformational transitions and free-energy landscape that underlie fidelity decisions in DNA synthesis. Ternary complexes of high fidelity derivatives with complementary dNTPs adopt mainly a fully closed conformation, whereas a conformation with a FRET value between those of open and closed is sparsely populated. This intermediate-FRET state, which we attribute to a partially closed conformation, is also predominant in ternary complexes with incorrect nucleotides and, strikingly, in most ternary complexes of low-fidelity derivatives for both correct and incorrect nucleotides. The mutator phenotype of the low-fidelity derivatives correlates well with reduced affinity for complementary dNTPs and highlights the partially closed conformation as a primary checkpoint for nucleotide selection.
ymerases depends on conformational changes that promote the rejection of incorrect nucleotides before phosphoryl transfer. Here, we combine single-molecule FRET with the use of DNA polymerase I and various fidelity mutants to highlight mechanisms by which active-site side chains influence the conformational transitions and free-energy landscape that underlie fidelity decisions in DNA synthesis. Ternary complexes of high fidelity derivatives with complementary dNTPs adopt mainly a fully closed conformation, whereas a conformation with a FRET value between those of open and closed is sparsely populated. This intermediate-FRET state, which we attribute to a partially closed conformation, is also predominant in ternary complexes with incorrect nucleotides and, strikingly, in most ternary complexes of low-fidelity derivatives for both correct and incorrect nucleotides. The mutator phenotype of the low-fidelity derivatives correlates well with reduced affinity for complementary dNTPs and highlights the partially closed conformation as a primary checkpoint for nucleotide selection.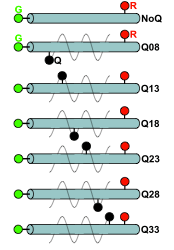 relax from the excited state to the ground state nonradiatively (i.e., are dark). As a result, they can serve as acceptors for Förster resonance energy transfer experiments without contributing significantly to background in the donor-emission channel, even at high concentrations. Although the advantages of dark quenchers have been exploited for ensemble bioassays, no systematic single-molecule study of dark quenchers has been performed, and little is known about their photophysical properties. Here, we present the first systematic single-molecule study of dark quenchers in conjunction with fluorophores and demonstrate the use of dark quenchers for monitoring multiple interactions and distances in multichromophore systems. Specifically, using double-stranded DNA standards labeled with two fluorophores and a dark quencher (either QSY7 or QSY21), we show that the proximity of a fluorophore and dark quencher can be monitored using the stoichiometry ratio available from alternating laser excitation spectroscopy experiments, either for single molecules diffusing in solution (using a confocal fluorescence) or immobilized on surfaces (using total-internal-reflection fluorescence). The latter experiments allowed characterization of the dark-quencher photophysical properties at the single-molecule level. We also use dark-quenchers to study the affinity and kinetics of binding of DNA Polymerase I (Klenow fragment) to DNA. The measured properties are in excellent agreement with the results of ensemble assays, validating the use of dark quenchers. Because dark-quencher-labeled biomolecules can be used in total-internal-reflection fluorescence experiments at concentrations of 1 μM or more without introducing a significant background, the use of dark quenchers should permit single-molecule Förster resonance energy transfer measurements for the large number of biomolecules that participate in interactions of moderate-to-low affinity.
relax from the excited state to the ground state nonradiatively (i.e., are dark). As a result, they can serve as acceptors for Förster resonance energy transfer experiments without contributing significantly to background in the donor-emission channel, even at high concentrations. Although the advantages of dark quenchers have been exploited for ensemble bioassays, no systematic single-molecule study of dark quenchers has been performed, and little is known about their photophysical properties. Here, we present the first systematic single-molecule study of dark quenchers in conjunction with fluorophores and demonstrate the use of dark quenchers for monitoring multiple interactions and distances in multichromophore systems. Specifically, using double-stranded DNA standards labeled with two fluorophores and a dark quencher (either QSY7 or QSY21), we show that the proximity of a fluorophore and dark quencher can be monitored using the stoichiometry ratio available from alternating laser excitation spectroscopy experiments, either for single molecules diffusing in solution (using a confocal fluorescence) or immobilized on surfaces (using total-internal-reflection fluorescence). The latter experiments allowed characterization of the dark-quencher photophysical properties at the single-molecule level. We also use dark-quenchers to study the affinity and kinetics of binding of DNA Polymerase I (Klenow fragment) to DNA. The measured properties are in excellent agreement with the results of ensemble assays, validating the use of dark quenchers. Because dark-quencher-labeled biomolecules can be used in total-internal-reflection fluorescence experiments at concentrations of 1 μM or more without introducing a significant background, the use of dark quenchers should permit single-molecule Förster resonance energy transfer measurements for the large number of biomolecules that participate in interactions of moderate-to-low affinity. transfer (FRET) efficiency are often used to study the structures of biomolecules and relate these structures to function. Methods like probability distribution analysis analyze FRET histograms to detect heterogeneities in molecular structure, but they cannot determine whether this heterogeneity arises from dynamic processes or from the coexistence of several static structures. To this end, we introduce burst variance analysis (BVA), a method that detects dynamics by comparing the standard deviation of FRET from individual molecules over time to that expected from theory. Both simulations and experiments on DNA hairpins show that BVA can distinguish between static and dynamic sources of heterogeneity in single-molecule FRET histograms and can test models of dynamics against the observed standard deviation information. Using BVA, we analyzed the fingers-closing transition in the Klenow fragment of Escherichia coli DNA polymerase I and identified substantial dynamics in polymerase complexes formed prior to nucleotide incorporation; these dynamics may be important for the fidelity of DNA synthesis. We expect BVA to be broadly applicable to single-molecule FRET studies of molecular structure and to complement approaches such as probability distribution analysis and fluorescence correlation spectroscopy in studying molecular dynamics.
transfer (FRET) efficiency are often used to study the structures of biomolecules and relate these structures to function. Methods like probability distribution analysis analyze FRET histograms to detect heterogeneities in molecular structure, but they cannot determine whether this heterogeneity arises from dynamic processes or from the coexistence of several static structures. To this end, we introduce burst variance analysis (BVA), a method that detects dynamics by comparing the standard deviation of FRET from individual molecules over time to that expected from theory. Both simulations and experiments on DNA hairpins show that BVA can distinguish between static and dynamic sources of heterogeneity in single-molecule FRET histograms and can test models of dynamics against the observed standard deviation information. Using BVA, we analyzed the fingers-closing transition in the Klenow fragment of Escherichia coli DNA polymerase I and identified substantial dynamics in polymerase complexes formed prior to nucleotide incorporation; these dynamics may be important for the fidelity of DNA synthesis. We expect BVA to be broadly applicable to single-molecule FRET studies of molecular structure and to complement approaches such as probability distribution analysis and fluorescence correlation spectroscopy in studying molecular dynamics.